PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
1.39 - 1.41
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
1.572 - 1.580
GENERAL DESCRIPTION AND APPLICATIONS
Tin compounds are classified into two main groups; inorganic-tin and organo-tin compounds. The organo-tin compounds are defined as compounds in which at least one tin-to-carbon bond exist. But the inorganic-tin compounds do not contain carbon as the principal element. Inorganic-tin compounds are relatively simple in their molecular structure and, like tin itself, are not considered to be toxic. Tin atoms can replace carbon atoms in chemical compounds, and a great variety of organo-tin compounds are known.
INORGANIC TIN COMPOUNDS
The largest use for inorganic tin compounds is in electrolytes for plating tin and tin alloys. The more important plating chemicals are chlorides, sulfates, and fluoroborates in acidic electrolytes and stannates in alkaline solutions. Inorganic-tin compounds are divided into two series: stannous, or tin(II), compounds and stannic, or tin(IV), compounds.Chemically, tin exhibits valencies of 2 and 4. It resists attack by water but is dissolved by strong acids and alkalis. One of common compounds of tin(II) are stannous chloride (SnCl2) used in tin galvanizing, as a reducing agent in the manufacture of polymers and as a mordant in dyeing.; stannous oxide (SnO) employed in making tin salts for chemical reagents and for plating; and stannous fluoride (SnF2) is the additive in fluoride tooth-pastes. Inorganic tin chemicals are used as catalysts in a number of industrial processes. stannous octoate is the catalyst that produces the foaming action that turns the liquid plastic into a foamlike solid structure in the manufacture of polyurethane foam. Tin(IV) compounds of significance include stannic chloride (SnCl4) is widely used as a stabilizer for perfumes and as a starting material for other tin salts; and stannic oxide(SnO2) is a useful catalyst in certain industrial processes and a polishing powder for steel. Tin sulfide is used as a bronzing agent for wood colouring
ORGANOTIN COMPOUNDS
- Tributyltin benzoate (CAS RN: 4342-36-3)
- Tributyltin chloride (CAS RN: 1461-22-9)
- Tributyltin fluoride (CAS RN: 1983-10-4)
- Tributyltin linoleate (CAS RN: 24124-25-2)
- Tributyltin methacrylate (CAS RN: 2155-70-6)
- Tributyltin naphthenate (CAS RN: 85409-17-2)
- Tributyltin oxide (CAS RN: 56-35-9)
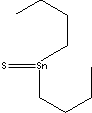
- Tributyltin sulfide (CAS RN: 4808-30-4)
- Tributyltin adipate (CAS RN: 7437-35-6)
- Tributyltin acetate (CAS RN: 56-36-0)
- Triphenyltin hydroxide (CAS RN: 76-87-9)
- Triphenyltin acetate (CAS RN: 900-95-8)
- Triphenyltin chloride (CAS RN: 639-58-7)
Tributyltin compounds are usually clear to yellowish liquids with an unpleasant odor. Triphenyltincompounds are white solids with low vapour pressures. Tri-organotin compounds are derivatives of tetravalent tin. They are lipophilic and have low water solubility. Physical and chemical properties of tri-organotin compounds vary depending upon the anion linked to tin. Tributyltin derivatives have toxic properties to gram positive bacteria are used as disinfectants on surfaces such as hospital floors and sports arenas, combined with gram negative bactericides. Tin chemicals also used as flame retardants to treat fabrics and plastics. Tributyltin methacrylate is used as a stabiliser for PVC. Other industrial applications of organotin compounds include as rodent repellents, antioxidants, curing agents and corrosion inhibitors.
Di-n-butyltin oxide is used as an intermediate for polyurethane catalysts and butyltin family stabilizers for polymers.
APPEARANCE
PURITY
98.0% min
43.0 - 45%